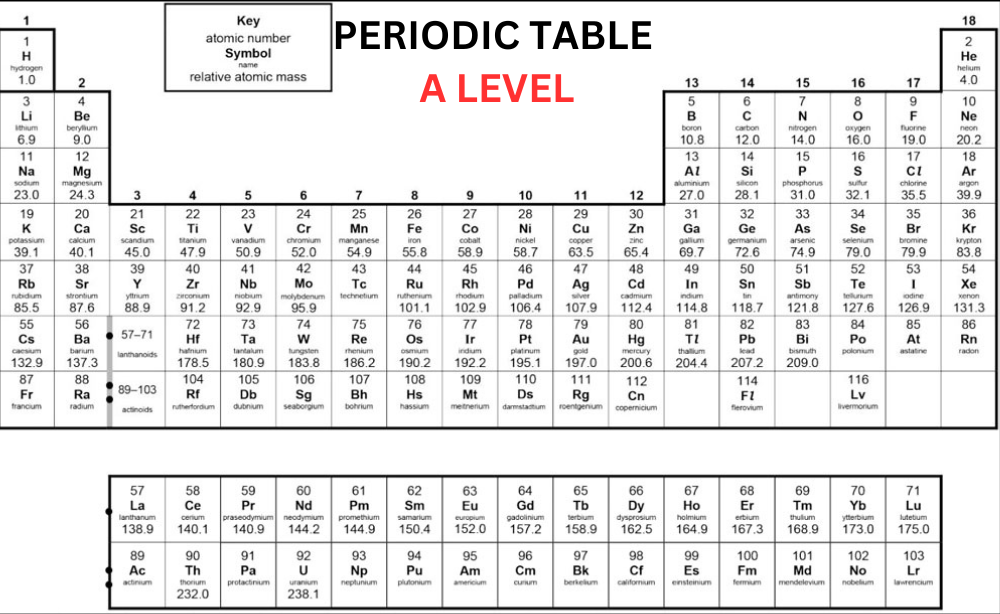
Old Periodic Table A Level
Dmitri Mendeleev (1834-1907) was a Russian chemist. In the 19th century, he was one of many scientists trying to organize all the elements known at the time. In 1869, Mendeleev created his first periodic table of the elements. He arranged the elements by their increasing relative atomic mass. Then, he looked at the properties of the elements and their compounds. Mendeleev noticed that doing this allowed him to group elements with similar properties together in columns. However, to make his table work, he had to leave gaps for elements that hadn’t been discovered yet and switch the order of a few pairs of elements.
Mendeleev left gaps in his table for elements that weren’t known yet. By checking the properties of elements surrounding these gaps, he could predict the features of the missing elements. For instance, he predicted ‘eka-silicon’, which later turned out to be germanium. The properties of germanium matched Mendeleev’s predictions, validating his periodic table concept.to understand A Level Periodic Table, you must understand it from a basic, that’s why we discussed the traditional periodic table before proceeding further to a modern periodic table. If you are still confused how periodic table is created, then you can ask A Level Chemistry Tutor at Bright Mind Tutors.
The Modern A Level Periodic Table
Now, let’s discuss the modern OCR periodic table:
- Atomic Number and Protons
In the beginning, an element’s atomic number was only determined by its position on the periodic table. However, after the discovery of protons, scientists identified that an element’s atomic number corresponds to the number of protons in its nucleus. In the contemporary Periodic Table A Level, elements are organized based on their atomic number rather than their relative atomic mass. In the periodic table, the elements are organized into:
- Horizontal rows, known as periods, which identify with the increasing atomic number
- Vertical columns are known as groups where the elements share the same properties.
- Group Numbers
The group numbers have been modified over time:
- In older periodic table, you could see groups number 1-7 and 0 are referred to as the main groups.
- On the other hand, while studying Periodic Table OCR A Level, you will notice that ‘IUPAC’ group numbers 1-18 are mentioned for all the groups. Hydrogen (H) is positioned at top of the Group 1, which is not a member of Group 1.
- Metals and Non-Metals in the Table
Metallic elements are positioned on the left side of the periodic table, while non-metallic elements occupy the right side. Visualizing a zigzag line, beginning with B-Al-Si, delineates the boundary between metals and non-metals.
| Metals |
Non-Metals |
| Shiny | Dull |
| High melting point | Low melting point |
| Good conductors of heat and electricity | Poor conductors of heat and electricity |
| High density | Low density |
| Malleable | Brittle |
- Resolving Pair Reversals
Mendeleev was not aware of isotopes, which later emerged as an explanation for the reversal of pairs in his table. The interchange of positions between iodine and tellurium in Mendeleev’s arrangement occurred because:
- Iodine, with its single naturally occurring isotope, 127
- Tellurium, on the other hand, is predominantly composed of isotopes 128Te and 130Te, which are more abundant.
The higher relative abundance of tellurium isotopes results in a greater relative atomic mass, despite iodine having a higher atomic number (53). Thus, in the modern A Level Periodic Table, tellurium (atomic number 52) precedes iodine (atomic number 53), aligning with the correct order of elements.
- Electron Arrangements
Various electron shells have distinct maximum capacities for holding electrons. Electrons first fill the innermost shell before moving to the next shell once the previous reaches its capacity. For elements with atomic numbers 1 to 20: the first electron shell has 2 maximum number of electrons, and second and third electron shells have 8 maximum number of electrons.
CLICK ON THIS LINK TO DOWNLOAD => Periodic Table A Level
Other Useful Links: