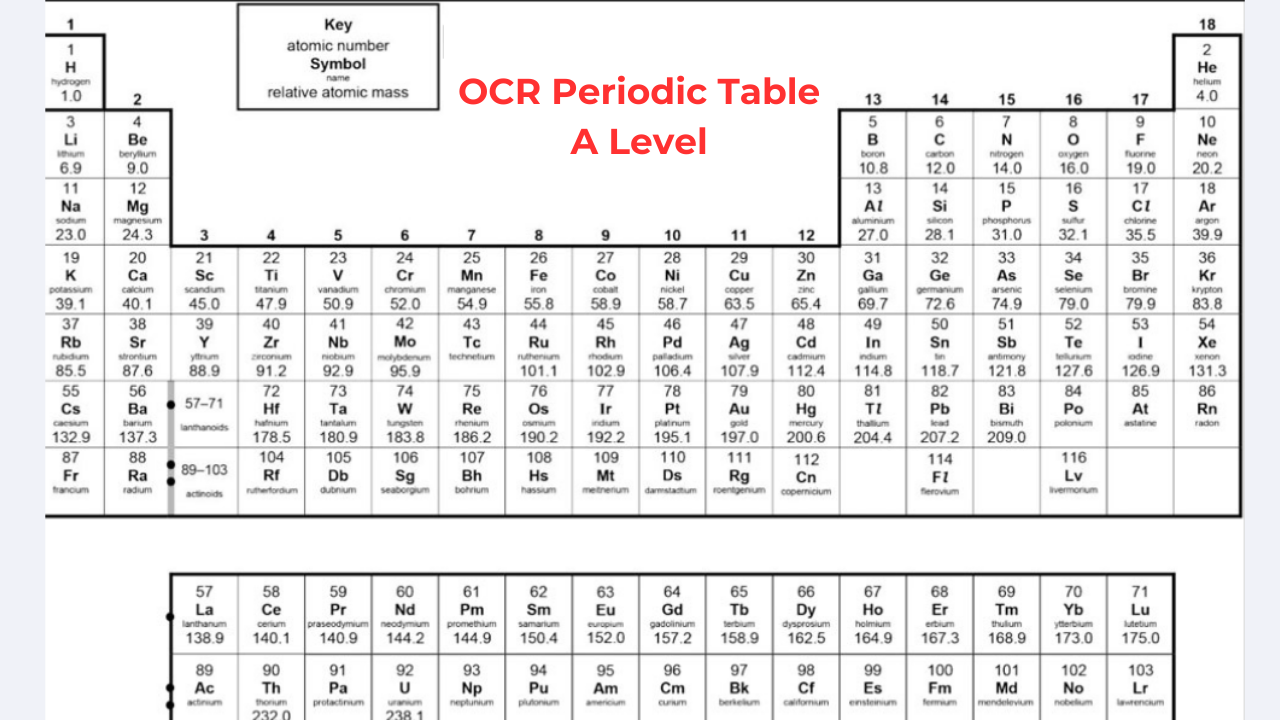
In this blog, we’ll discuss OCR A Level Periodic Table in a simple way. Let’s get started.
History of the Periodic Table A Level OCR
Before the discovery of protons, neutrons, and electrons, scientists organized elements by atomic weight. Early periodic tables had errors and gaps because they relied solely on atomic weights. Mendeleev improved the periodic table by leaving gaps for elements he predicted hadn’t been discovered yet and adjusting the order based on atomic weights. He became famous for accurately predicting the properties and atomic weights of these missing elements. Later discoveries confirmed his predictions, and the understanding of isotopes explained why atomic weights alone were not always accurate. If you find it difficult to understand it, you can get in touch with A Level Chemistry Tutor at Bright Mind Tutors.
OCR A Level Periodic Table
The periodic table arranges elements so that those with similar properties are in the same columns, called groups. For example, Group 1 metals are very reactive with water and share the same number of electrons in their outer shell. Elements in the same row have a similar number of electron shells. In the A Level chemistry periodic table, metals are on the left, while non-metals are on the right. Metals typically have 1, 2, or 3 electrons in their outer shell and tend to lose them to form positive ions. Non-metals usually have 5, 6, or 7 electrons in their outer shell and tend to gain electrons to form negative ions. Noble gases have 8 electrons in their outer shell and are stable, so they don’t form ions.
Group 0 (Noble Gases)
Noble gases like neon, helium, xenon, and krypton have full outer electron shells, making them very stable and non-reactive. Helium has 2 electrons in its outer shell, while the others have 8. These colorless gases have low boiling and melting points and are gases at room temperature. The boiling points increase down the group due to stronger intermolecular forces requiring more energy to break. Despite A-level chemistry, if you find GCSE biology difficult, you can reach our GCSE Biology Tutor at Bright Mind Tutors.
Group 1 (Alkali Metals)
Lithium, sodium, potassium, rubidium, and cesium are in Group 1, known as alkali metals. They each have one electron in their outer shell and are highly reactive, especially with air and water. These metals are soft, can be cut with a knife, and have relatively low melting points that decrease down the group. Due to their reactivity, they are stored in oil to prevent oxidation.
Group 7 (Halogens)
Fluorine, chlorine, bromine, and iodine are in Group 7, known as halogens. Each has 7 electrons in their outer shell. As you move down the group, the elements change from gases to solids, with increasing melting and boiling points due to larger molecules and stronger intermolecular forces. Reactivity decreases down the group because the larger atomic size weakens the attraction for electrons. Elements at the top, like fluorine and chlorine, are more reactive than those at the bottom, like iodine and astatine.
For more information about periodic table, you can book a free trial session with our A Level Chemistry Tutor Online at Bright Mind Tutors.
Other Useful Links: