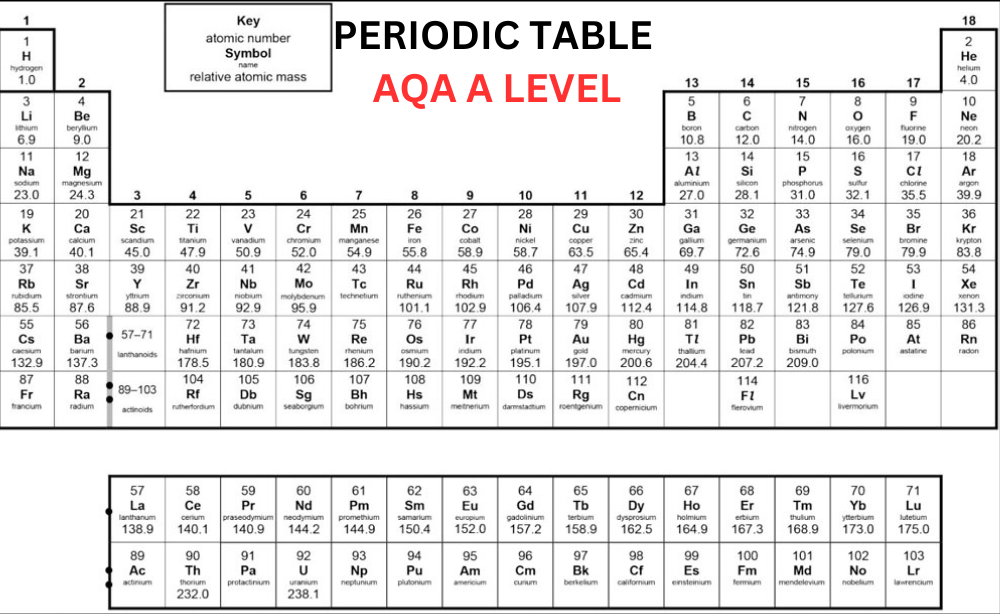
The chemists have arranged the known elements on Earth, called the periodic table. The elements are organized in columns, known as groups – all elements in the same group will be part of the same type of chemical reaction. Today, we’ll discuss the basic concept of A Level Periodic Table. Learn Periodic Table in detail from A Level Chemistry Tutor Online at Bright Mind Tutors.
What is Periodic Table A Level?
Elements in the periodic table are organized in rows, called periods in an increasing order of atomic number with each successive element having one additional proton as compared to the element before them. When you search for AQA Periodic Table A Level, you will come to know that the table is organized in columns, known as groups consisting of elements that have the same chemical properties. The groups are numbered from 1 to 8, skipping the section of elements in the middle of the table.
You can see in the Periodic Table AQA A Level that elements in the same group in the table have the same number of electrons in their outer shell and it gives them similar chemical properties. For instance, fluorine is in group 7 of the periodic table so we know that it has 7 electrons in its outer shell. Other elements in group 7 include bromine, chlorine, and iodine – all these elements share similar properties.
The majority of the elements on the A Level Periodic Table are metallic, occupying the left side, while non-metallic elements are situated on the right side. The arrangement of elements in the periodic table allows us to anticipate the reactions they will undergo:
Elements found in groups 1 and 2, possessing 1 or 2 electrons to lose for achieving a full outer shell, will typically interact with elements in groups 6 or 7 when forming compounds. Group 8 elements, known as noble gases, remain unreactive with other elements due to their complete octet configuration.
Group 0
Group 0, also known as group 8 sometimes, is the last group on the Periodic Table A Level and consists of noble gases. These gases, like helium, neon, and argon, have a full outer shell of eight electrons (except helium, which has only two electrons). Because they have this complete outer shell, they don’t react with other elements to form molecules or compounds.
As you move down group 0, from helium to radon, the boiling point rises. This happens because these elements gain more electrons as you go down the group. With more electrons, they develop stronger intermolecular forces. These stronger forces need more energy to break when the element changes from a liquid to a gas.
Group 1
Sodium, lithium and potassium all react with water so that it can generate a metal hydroxide and hydrogen. The metal hydroxide is an alkali that releases hydroxide ions (OH-) into the solution. As all metals in group 1 react with water to create alkalis, they are known as ‘alkali metals’.
Group 7
You can see the halogen elements that are present in the second-to-last group of the AQA Periodic Table A Level. They have 7 electrons in their outer shell and they need only one more so that they can complete their octet. The halogens commonly react with elements in group 1 to form ionic compounds because the group 1 elements possess one outer electron, which the group 7 elements tend to acquire.
Conclusion
It is crucial to prepare A Level Chemistry Periodic Table when preparing for the exam. You can also receive support from tutors at Bright Mind Tutors. You can also book a free trial with us so you can decide if you like our tutoring services.
Other Useful Links: