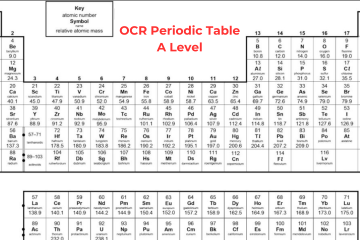
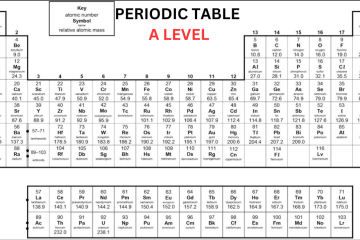
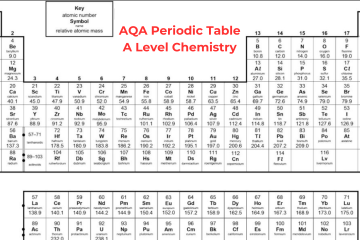
A Level Chemistry Periodic Table OCR
History of Periodic Table
Before scientists knew about protons, neutrons, and electrons, they tried to organize elements based on their atomic weights. However, the early periodic tables had gaps and mistakes because they only used atomic weights. Mendeleev fixed some of these problems by leaving gaps for elements he thought hadn’t been found yet and, in some places, he changed the order based on atomic weights. He became famous for these gaps, saying they were for elements that hadn’t been discovered. He even guessed the properties and weights of some of these missing elements correctly! Later, scientists found elements that fit Mendeleev’s predictions and filled the gaps. Understanding isotopes helped explain why atomic weights alone weren’t always right.
Learn Periodic Table in detail from A Level Chemistry Tutor at Bright Mind Tutors.
OCR A Level Chemistry Periodic Table
The Periodic table is organized in a way that elements with the same characteristics can be found together in columns, known as groups – for instance, the group 1 metals are very reactive with water. Elements in the same group consist of the same number of electrons in their outer shell. Elements in the same row have a similar number of electron shells. A level chemistry periodic table OCR shows that it is split with metals all present on the left side of the table and non-metals on the right side. Metals have either 1, 2, or 3 electrons on their outermost shell, to lose electrons to create positive ions. Non-metals that consist of either 5, 6, or 7 electrons on their outermost shell want to obtain electrons to create negative ions. Non-metals with 8 electrons, the noble gases, don’t obtain or lose electrons to create ions, as they are stable.
Group 0 (Noble Gases)
Neon, helium, xenon, and krypton are all noble gases because they contain full outer shells of electrons and they are stable. It means they are not reactive as they don’t require to react to obtain electrons – so they are known as inert. Except for helium, the noble gases contain 8 electrons in their outer shell as helium has only 2 electrons. These gases are colourless. All elements in this group are gases at room temperature as they have very low boiling and melting points. The boiling points increase down the group and it is because of stronger intermolecular forces of attraction, i.e. more energy is required to break them.
Group 1 (Alkali Metals)
Lithium, sodium, potassium, rubidium, and caesium all belong to Group 1 and are commonly referred to as the Alkali Metals. Each atom of these elements has a single electron in its outermost shell. These metals have some unique characteristics as they:
- Are highly malleable and can be easily cut with a knife.
- Have relatively low melting points, which decrease as you move down the group.
Due to their high reactivity with air, these metals must be stored in oil to prevent oxidation. This is evident when the metal is cut: the inside appears shiny while the outer surface appears dull. When Group 1 metals react with water, they produce an alkaline solution.
Group 7 (Halogens)
Fluorine, chlorine, bromine, and iodine belong to Group 7 and are commonly referred to as Halogens. Each atom of these elements has 7 electrons in its outermost shell. Notice the transition from the uppermost element of the group (fluorine, a gas) to the lowermost (astatine, a solid), one notices a significant increase in the melting and boiling points of these elements. This change occurs because:
- The size of the molecules increases.
- The intermolecular forces between molecules strengthen.
- Consequently, more energy is required to overcome these forces.
As you move down Group 7, the elements become less reactive due to their larger atom size. Elements at the top (like fluorine, and chlorine) are more reactive than those at the bottom (like iodine, and astatine). This is because the outer electron shell, being farther from the nucleus, experiences weaker attractive forces, making it harder to attract an electron.
Please check the below-mentioned OCR A Level Chemistry Periodic Table PDF as a guide for your exam preparation.
CLICK ON THIS LINK TO DOWNLOAD => OCR A Level Chemistry Periodic Table PDF
Other Useful Links:


This periodic table is very informative. Thank you for posting it!