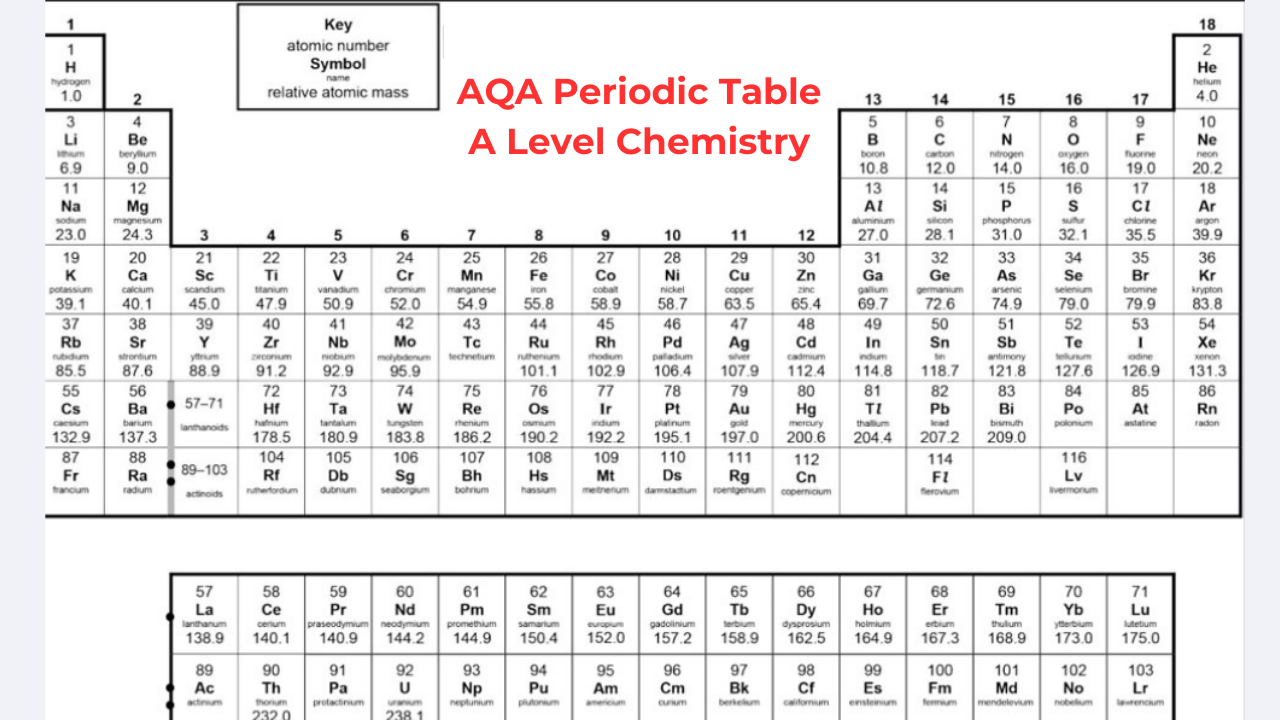
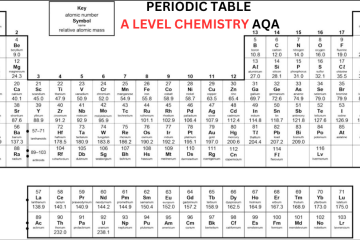
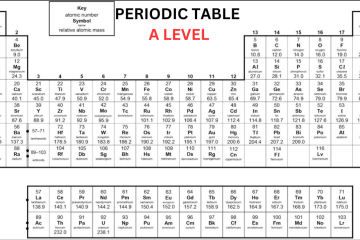
When you come across AQA Periodic Table A Level Chemistry, you will come to know that the periodic table in AQA A-Level Chemistry is arranged in rows called periods and columns called groups. According to the increasing atomic number, the elements are organized. Each successive element within a row consists of one additional proton compared to the preceding element. The table is organized in such a way that elements sharing similar chemical properties are added to the same group. The groups are numbered one through eight, excluding the central block of elements. You can learn more about the periodic table by receiving help from A Level Chemistry Tutor Online at Bright Mind Tutors.
Periodic Table A Level Explained
Elements within the same group contain the same number of electrons in their outer shell, which results in analogous chemical characteristics. For example, fluorine belongs to group 7, indicating that it has seven electrons in its outer shell. Similarly, other members of group 7, such as chlorine, bromine, and iodine, show comparable properties and react in similar ways.
The majority of A Leve Periodic Table consists of metallic elements, which are arranged predominantly on the left side, while non-metallic elements are predominantly arranged on the right side. The arrangement of elements in the periodic table makes it easy to assess the reactions they are likely to undergo. Elements in groups 1 and 2 typically react with elements from groups 6 or 7 when creating compounds. Elements in group 8, known as noble gases, are not likely to react with other elements due to their possession of a complete octet.
History: Periodic Table A Level Chemistry
Before the discovery of subatomic particles, elements were arranged by atomic weight. Dmitri Mendeleev left gaps and rearranged elements for better grouping. Later, discoveries filled these gaps by finding elements that fit in these gaps, and understanding isotopes clarified element placement. Today, the periodic table is organized by increasing atomic number.
Group 0 in AQA Periodic Table A Level
The last group of the periodic table is Group 0, also known as Group 8, consisting of noble gases. These elements are considered gases at room temperature and they consist of a full outer shell of eight electrons, except for helium which has two electrons. Because they have a full outer shell, they are unreactive and do not create molecules or compounds.
As we move down Group 0, from helium to radon, the boiling point increases. This is due to the fact that the elements have more electrons, leading to stronger intermolecular forces. These stronger forces need more energy to overcome when converting from a liquid to a gas state.
Group 1 in A Level Periodic Table AQA
Lithium, sodium, and potassium, which are in Group 1, react strongly with water, forming a metal hydroxide and hydrogen gas. The metal hydroxide is an alkali, which means it releases hydroxide ions (OH-) into the solution. Since all group 1 metals react this way to form alkalis, they are known as “alkali metals.” As we move down Group 1, metals become more reactive. Sodium and Lithium are quite reactive, but potassium is more reactive, often resulting in explosive reactions. Elements below potassium seen in Periodic Table AQA A Level are even more reactive but are rare and found in small amounts.
Group 7 in AQA Periodic Table A Level Chemistry
The halogens are elements arranged in the second-to-last group of the AQA A-Level Chemistry periodic table, called Group 7. 7 electrons are present in outer shell, making them reactive as they only need one more to complete their octet. Halogens usually create ionic compounds with elements in Group 1, which have one outer electron that the Group 7 elements are likely to take.
As we move down the halogens, from fluorine to astatine, they become darker in color and have higher boiling points. This occurs because each element’s mass increases, and they have more electrons around their nuclei. More electrons mean more intermolecular forces can form.
Conclusion
You can get in touch with Bright Mind Tutors where we help students get detailed information regarding each concept of A Level Chemistry.
Other Useful Links: