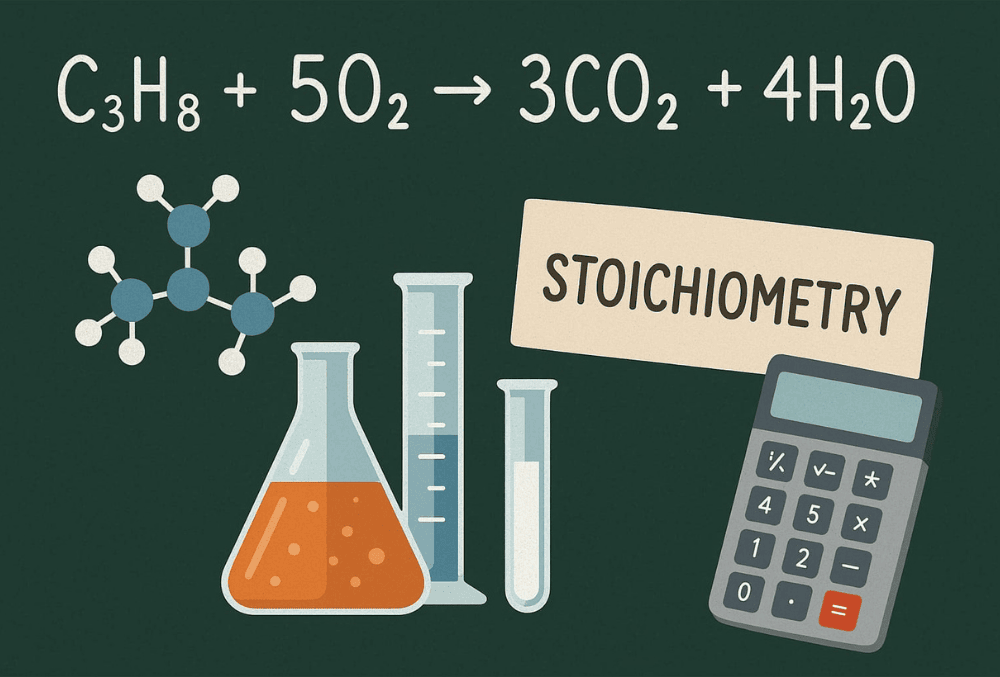Balancing chemical equations and solving stoichiometry problems are foundational skills in AP Chemistry. These concepts not only show up frequently on the AP exam but also form the base for nearly every reaction-related topic you will encounter in the course.
In this blog by BrightMind Tutors, we’ll walk through key concepts, formulas, and strategies for success in balancing equations and solving stoichiometry problems — helping you master the material with clarity and confidence. Whether you’re studying independently or working with an AP Chemistry tutor, this guide is designed to make complex topics more approachable.
What is a Chemical Equation?
A chemical equation represents a chemical reaction where the reactants are transformed into products. For example:
2H2 + O2 → 2H2O
Here, hydrogen and oxygen are reactants, and water is the product. Balancing these equations ensures the Law of Conservation of Mass is obeyed. The number of atoms for each element must be the same on both sides of the equation.
Chemical equations also give insight into the physical states of the reactants and products. For example:
2H2(g) + O2(g) → 2H2O(l)
This notation tells you that hydrogen and oxygen are gases and water is a liquid.
Why Balancing Equations Matters in AP Chemistry
Balancing equations is not just an academic exercise — it’s essential for real-world chemistry and exam success. Here’s why:
- It’s frequently tested in both MCQs and FRQs.
- Balancing is a prerequisite for stoichiometric calculations.
- Unbalanced equations give wrong mole ratios, leading to calculation errors.
- It ensures correct proportions of reactants and products in lab experiments.
- It helps in identifying limiting and excess reagents.
In AP Chemistry, reaction-based problems often begin with an unbalanced equation. You must balance it before doing any quantitative calculations, or you’ll lose accuracy.
Steps to Balance a Chemical Equation
- Write the unbalanced equation.
- List the number of atoms of each element on both sides of the equation.
- Balance atoms one at a time, usually starting with metals, then non-metals, and hydrogen/oxygen last.
- Use coefficients (not subscripts) to balance atoms. Coefficients multiply the number of atoms.
- Double-check that each element is balanced.
- Ensure all coefficients are in the lowest whole-number ratio.
Example: Unbalanced: C3H8 + O2 → CO2 + H2O Balanced: C3H8 + 5O2 → 3CO2 + 4H2O
Practice Tip: Start by balancing carbon atoms (C3), then hydrogen (H8), and finally oxygen.
Tips for Faster Balancing
- Balance polyatomic ions as a unit if they appear unchanged on both sides.
- Always simplify coefficients to the lowest whole numbers.
- Save balancing hydrogen and oxygen for last — especially in combustion reactions.
- Double-check by counting each type of atom.
- Memorize common diatomic elements (H2, O2, N2, F2, Cl2, Br2, I2).
Introduction to Stoichiometry
Stoichiometry is the area of chemistry that deals with quantitative relationships in chemical reactions. In AP Chemistry, stoichiometry is vital for:
- Predicting how much product will form
- Determining the limiting reagent
- Calculating percent yield
- Understanding real-life chemical quantities in lab situations
Key Concepts:
- Mole Ratio: The ratio between moles of different substances in a balanced equation
- Limiting Reagent: The reactant that is completely consumed first
- Theoretical Yield: The maximum amount of product expected
- Percent Yield: A measure of reaction efficiency
Common Stoichiometry Questions on AP Chemistry Exam
- Given mass A, find mass B
- Given moles A, find volume of gas B
- Determine the limiting reagent
- Calculate the percent yield
- Find the excess reagent left after the reaction
- Calculate the amount of product formed under non-STP conditions
Mole-to-Mole Conversions
This is the simplest type of stoichiometry problem.
Example:
N2 + 3H2 → 2NH3
If you have 4 mol of H2, how many mol of NH3 are produced? Use the mole ratio:
Mass-to-Mass Conversions
These require molar mass and a balanced equation.
Example:
Fe2O3 + 3CO → 2Fe + 3CO2
How many grams of Fe will be produced from 50 g of Fe2O3?
- Molar mass of Fe2O3 = 159.7 g/mol
- Molar mass of Fe = 55.85 g/mol
- Calculate moles of Fe2O3
- Apply mole ratio: 1 mol Fe2O3 → 2 mol Fe
- Convert to grams of Fe
Volume and Gas Stoichiometry
Use 22.4 L/mol at STP (Standard Temperature and Pressure) or ideal gas law if not at STP.
Problem Example: How many liters of O2 are required to burn 5.0 g of CH4 at STP?
- Write balanced equation: CH4 + 2O2 → CO2 + 2H2O
- Convert g CH4 to mol CH4
- Use mole ratio to find mol O2
- Convert mol O2 to volume: mol × 22.4 L
Limiting Reagent & Excess Reactant
If two reactants are given, identify which one limits the reaction.
Steps:
- Convert both reactants to moles
- Use mole ratio to determine product formation
- The smaller result = limiting reagent
This is one of the most frequently tested skills on the AP Chemistry FRQ section.
Percent Yield Problems
Used in lab-based FRQs or experimental-based MCQs.
Formula:
Percent Yield = (Actual Yield / Theoretical Yield) × 100
Example: Expected = 6.0 g; Actual = 5.1 g
(5.1 / 6.0) × 100 = 85% yield
AP Chemistry Exam Tip: Units Matter
Never skip units — always write units for:
- Moles (mol)
- Mass (g)
- Volume (L)
- Molar Mass (g/mol)
- Pressure (atm, mmHg)
Using units helps prevent calculation mistakes and can gain you partial credit in free-response questions.
Common Mistakes to Avoid
- Confusing subscripts with coefficients
- Skipping unit conversions (g to mol, mol to L)
- Not identifying the limiting reagent
- Using incorrect mole ratios
- Ignoring significant figures and rounding too early
Real AP Exam Practice Example
4Fe + 3O2 → 2Fe2O3
Question: If 20.0 g of Fe reacts with 12.0 g of O2, what is the limiting reactant and how much Fe2O3 is formed?
Solution Steps:
- Convert Fe and O2 to moles
- Use mole ratio to determine limiting reagent
- Use limiting reagent to calculate theoretical yield of Fe2O3
- Convert mol Fe2O3 to grams
Practice Strategy
- Practice at least 10 stoichiometry problems per week
- Use College Board’s AP Chemistry question bank
- Simulate exam conditions — time yourself
- Join BrightMind’s group review sessions
Work with a dedicated AP Chemistry tutor
Why BrightMind Tutors is Ideal for Stoichiometry Mastery
- Experienced AP Chemistry tutors with proven success
- Personalized 1-on-1 sessions
- Worksheets on every reaction type
- Mock FRQs and stepwise grading
- Live feedback and problem-solving breakdowns
- Monthly progress tracking and improvement plans
Conclusion
Balancing chemical equations and solving stoichiometry problems might seem intimidating at first, but with the right approach and consistent practice, they become second nature. These topics are a major portion of the AP Chemistry exam and mastering them gives you a solid score advantage.
If you’re struggling with the basics or want to boost your confidence before the exam, connect with an AP Chemistry tutor at BrightMind Tutors today. From mastering mole ratios to understanding limiting reagents and predicting product yields, we’ve got you covered.
Stay balanced. Stay focused. And remember: every mole counts!